
SCIENCE TECH INSTITUTE
Registered By GOVT. UP Higher Education Dept. & NO. 8 of 2002
Run By: Manraj Kuwar Singh Educational Society (REG NO. LUC/03140/2019-20)
Call Now - +91 8299547952

ONE WEEK ONLINE TRAINING COURSE ON BIOETHICS & GOOD CLINICAL PRACTICES (GCP) (Oct 23-29, 2025)

1. For Indian Participant: 600/- (Non-Refundable)
2. For Foreigner Participants registration here: $10/- (Non-Refundable)
About The Training
Good Clinical Practices (GCP) refer to a set of ethical and scientific quality standards for designing, recording and reporting trials that involve the participation of human subjects. GCP guidelines define the responsibilities of sponsors, investigators, and ethics committees in conducting clinical research. These guidelines are essential to maintaining the rights, safety, and well -being of clinical trial participants and ensuring the validity and reliability of clinical trial data. Everyone involved in the conduct of clinical research must be competent to perform their tasks, qualified by education, training and experience. This training course will cover all aspects of GCP guidelines and ethical issues in conducting clinical research.
OBJECTIVE OF THE TRAINING COURSE:
An understanding of GCP is prerequisite for anyone carrying out, or involved in, clinical research and clinical trials.
Members of ethics committees, institutional review boards (IRBs), or research ethics boards (REBs) who review and approve clinical trial protocols should have a solid understanding of GCP principles. GCP training helps them evaluate the ethical aspects of proposed trials, ensuring participant rights, safety, and welfare.
WHY THIS TRAINING COURSE IS IMPORTANT FOR YOU?
GCP training course is now essential for all PG students and teaching faculties as per recent NMC Guidelines.
ICMR guideline also states that the members of IRB and Independent Ethics Committee (IEC) should have GCP training.
THE EXPECTED OUTCOME OF THE TRAINING COURSE:
Empower clinicians and researchers about basics of GCP and aware about current regulation and guidelines in India for Clinical Trials
Understand the key components of GCP including informed consent research protocol
Ensure the data management and quality assurance policies and practices involved in clinical research
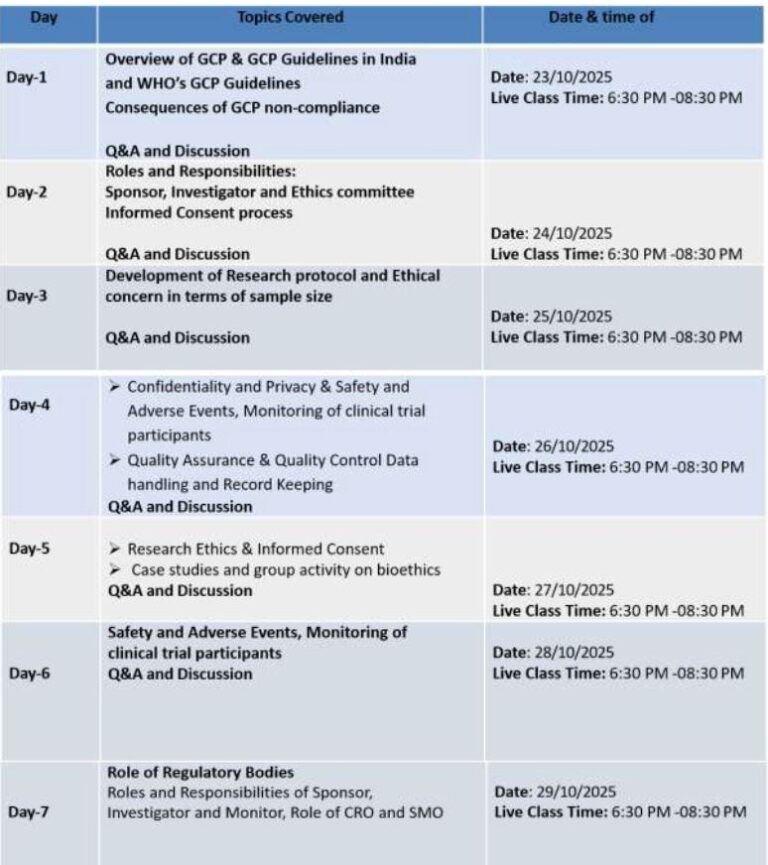
One Week Date: 23 to 29 Oct, 2025.
Course Rule
1.Registered participants will get e-certificates mentioned with 10 Credit Hours by HMC.
2.Study material in the form video recording of all sessions will be provided to all registered participants.
3.Training by Subject Experts from and other reputed Institute & Universities
4Training will be on the Zoom meeting platform.
5.Panel discussion after and before live class.


bank account details
Account Name: MKSES
Account Number: 683420110000456
Bank & Branch: BANK OF INDIA LDA COLONY LUCKNOW
IFSC: BKID0006834
MICR Code: 226013029
For More details
Training Course Co-ordinator
Dr. S.K Singh
Mob. No. +(91)8299547952,
WhatsApp No. +(91)8299547952 ,
Office Land line No. +(91 5223587193
Email: contact@sciencetechinstitute.com